Click here for details
Dissolved Oxygen & Oxygen Saturation
Dissolved Oxygen
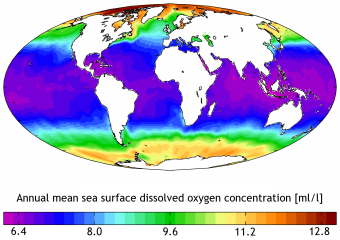
Oxygen concentrations in seawater are affected by exchanges with the atmosphere and by biological/chemical processes. The addition of oxygen can result from diffusion or mixing from the atmosphere or from photosynthesis. Oxygen is a byproduct of photosynthetic reactions carried out by seagrass, algae, and phytoplankton, which release it into the surrounding water. Oxygen is consumed through the biological processes of respiration (including plant respiration) and decomposition and by oxidation reactions with elements such as nitrogen, iron and sulfur.
| Oxygen + | Oxygen - |
|---|---|
| Photosynthesis | Respiration |
| Wave action | Decomposition |
| Lower temperatures | Higher temperatures |
| Lower salinity | Higher salinity |
| Clear water | Murky water |
The daily range (difference between high and low concentrations) of DO is low in oligotrophic (low nutrient) areas and high in eutrophic systems. If time averaged DO concentration is low, this is an indicator of high organic inputs, high pollutant inputs and high producer biomass.
Mobile animals will try to find higher DO concentrations when levels fall below 3 mg/l. Sardines die when concentrations fall to 2.2 mg/l. Hypoxic conditions occur when DO falls below 2 mg/l and many species are affected. Anoxia (no oxygen) is a term for when there is no dissolved oxygen and most organisms die. The Gulf of Mexico has a "dead zone" of seasonally hypoxic water that is caused by excessive nutrient loadings. The season is spring to summer when the winter supply of nutrients is taken up by phytoplankton with longer daylight hours. Estuaries are also susceptible to hypoxia because the fresh water at the surface forms a lens that does not readily mix with the saltier water underneath.
Recent Dissolved Oxygen Measurements
Oxygen Saturation
Oxygen saturation is the maximum amount of oxygen which can be retained in water without loss to the atmosphere. It is reported by SCCF RECON as the number of milligrams of oxygen per one liter of water. The solubility of gases, including oxygen, generally decreases with increasing temperature and salinity, and increases with increasing pressure.
